THERAPEUTIC EXPERTISE
Solutions for Oncology Research
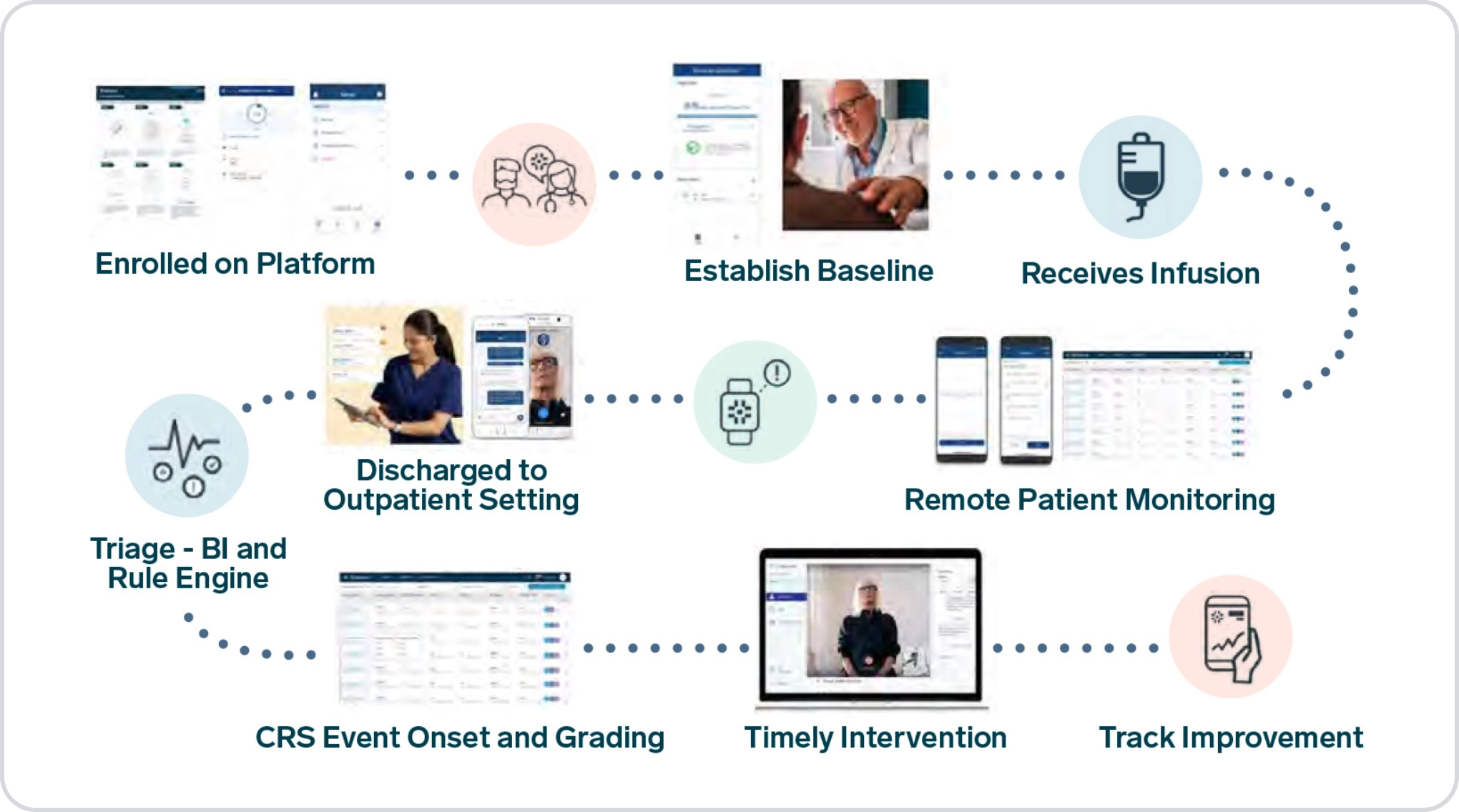
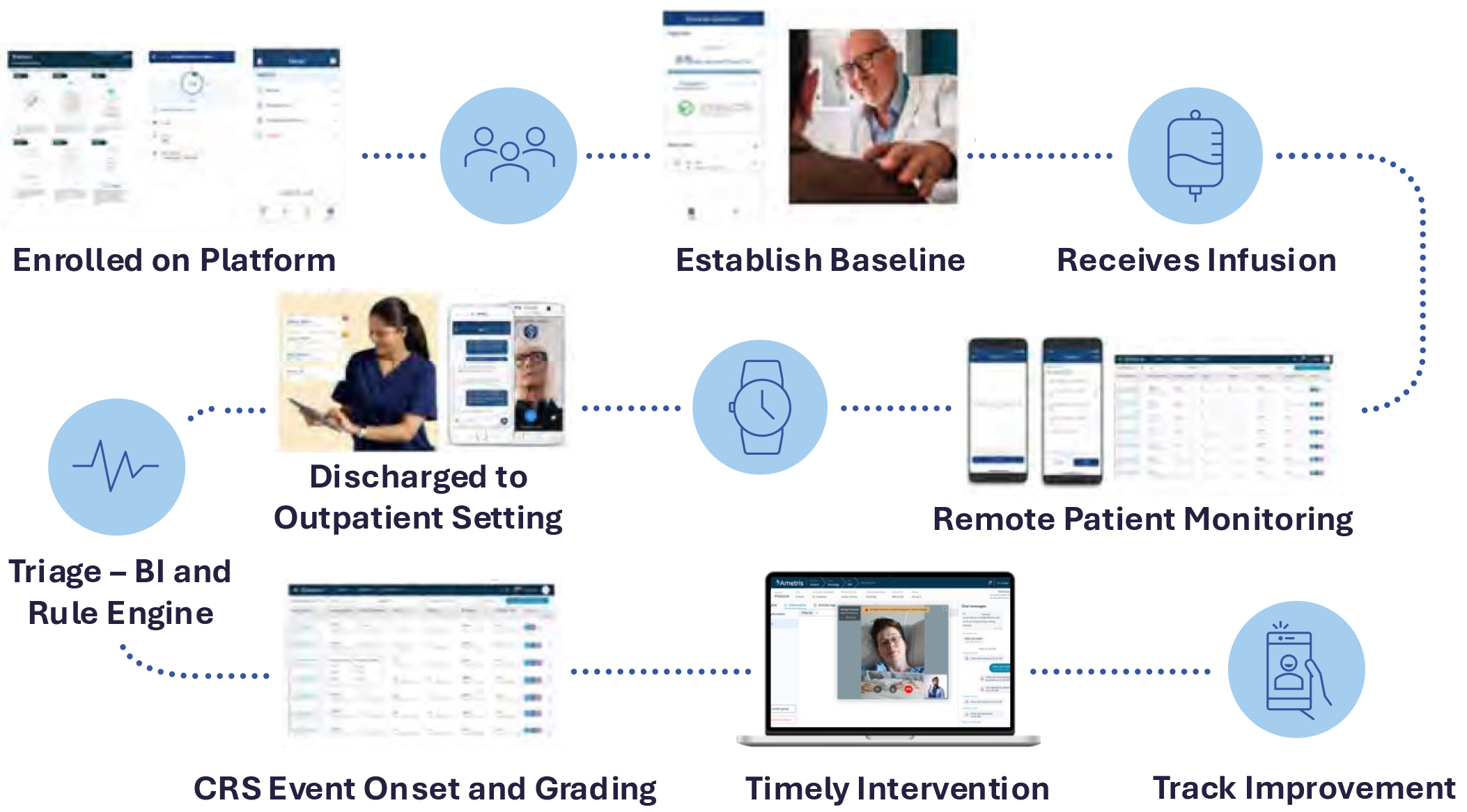
Digital health technologies empower the much-needed transformation of oncology drug development. DHTs can enable more effective trial designs that focus on the patient, improving the patient-centric nature of oncology trials, including immunotherapy treatments such as CAR-T cell therapy and trials investigating associated conditions such as cancer pain and cancer cachexia.
Schedule a Meeting Read Case Studies