 Connect™ Platform
Connect™ Platform
Achieve Precision at Every Phase of Clinical Development
Ametris Connect is a unified, end-to-end digital health technology (DHT) platform.
Schedule a MeetingNOTICE: Our office will be closed Monday, Jan 19th in observance of Martin Luther King Jr. Day. We will reopen at regular business hours on Tuesday, Jan 20th.
Ametris Connect is a unified, end-to-end digital health technology (DHT) platform.
Schedule a Meeting

The Ametris Connect platform combines validated clinical sensors, state-of-the-art algorithms, ePRO and remote data capture solutions, and seamless study operations to power continuous, patient-focused data collection across decentralized, hybrid, and traditional clinical trials.

Ecosystem of validated clinical sensors and wearables to support fit-for-purpose continuous and episodic physiologic monitoring and data capture.
Raw sensor data is transformed into validated digital measures with a comprehensive library of algorithms and expert data science support.
Centralized study oversight with tools for enrollment, onboarding tasks, and remote participant monitoring for sites and sponsors.
End-to-end DHT operations support includes project management, site training, logistics management, and clinical data operations and management.
Remote data capture and study management solutions, including eConsent, ePRO, and virtual visits, for decentralized and hybrid trials.
Our device-inclusive technology ecosystem integrates a variety of clinically validated sensors and wearables, enabling precise, protocol-aligned measurement across key physiological and functional domains, including activity, mobility, sleep, vital signs, cardiac and pulmonary function, involuntary movement, and cardinal disease symptoms. This flexibility allows study teams to design assessments around scientific objectives rather than device constraints.

Capture continuous and episodic data streams to support meaningful digital outcomes and biomarkers.
Manage deployment and data collection from multiple sensor modalities in a single platform, reducing site burden and simplifying workflows.
Combine objective sensor data with ePROs for a more complete view of participant status and disease progression.
Ametris Connect supports a growing library of pre-validated, fully integrated devices and sensors spanning multiple modalities and form factors, for both continuous and episodic data collection.
| Sensor / Modality | Digital Measures Availability | |
|
ActiGraph LEAP® |
||
|
ECG Patch |
|
|
|
Blood Pressure Cuffs |
|
|
|
Digital Spirometer |
|
|
|
Pulse Oximeter |
|
|
|
Wrist-worn Devices |
|
|
|
Digital Weight Scale |
|
|
View Full List of Digital Measures available with the ActiGraph LEAP
Don’t see what you’re looking for? Our platform is set up for rapid integration of new sensors and devices, and our in-house data science team supports the development of novel algorithms and digital endpoints.
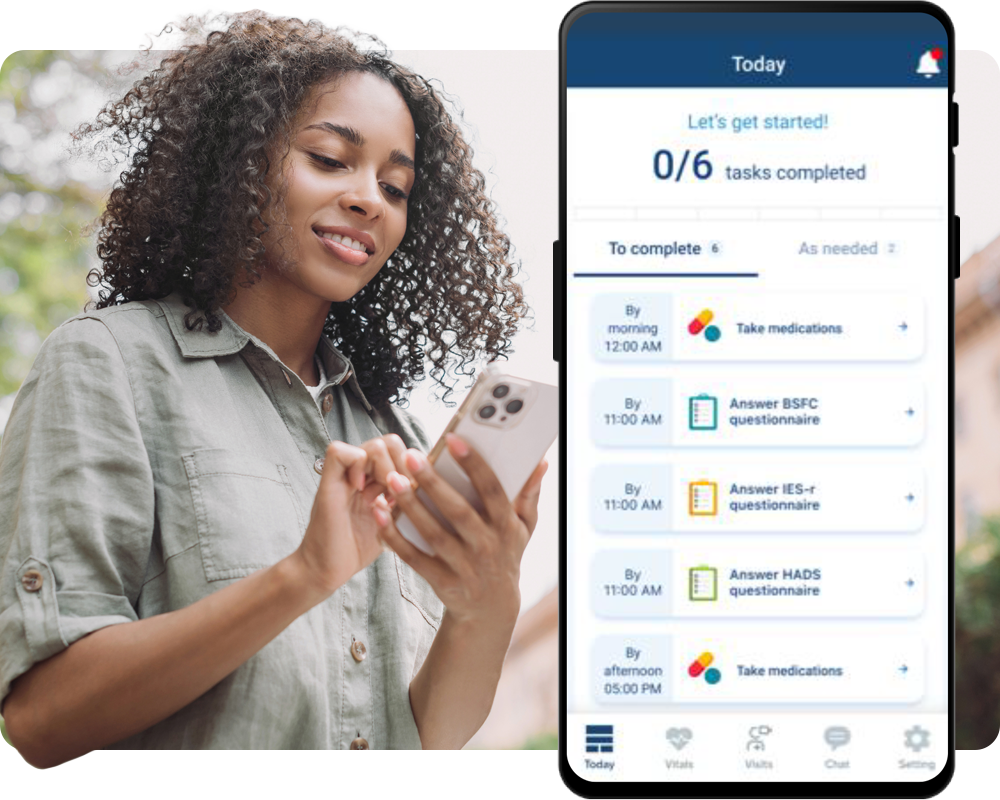
Empower participant engagement and improve data quality and completeness with an easy-to-use patient-facing mobile app.
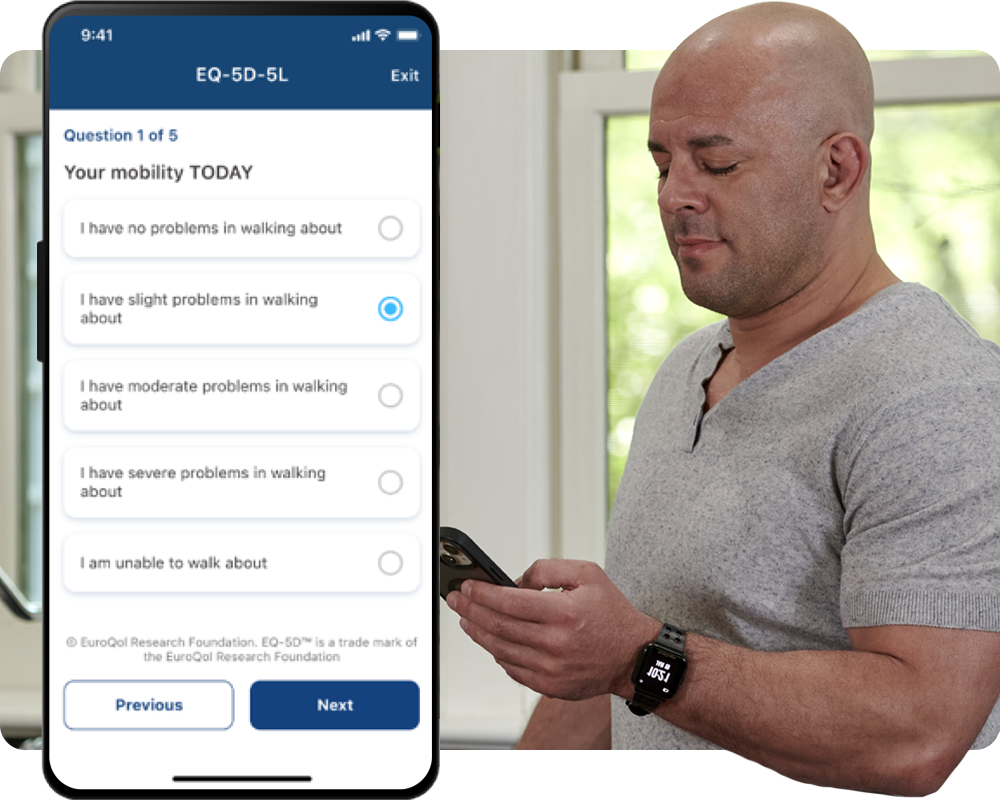
Easily deploy, scale, and capture clinically validated electronic patient-reported outcomes (ePRO) instruments across multiple platforms to enhance sensor-based digital measures in your clinical study.
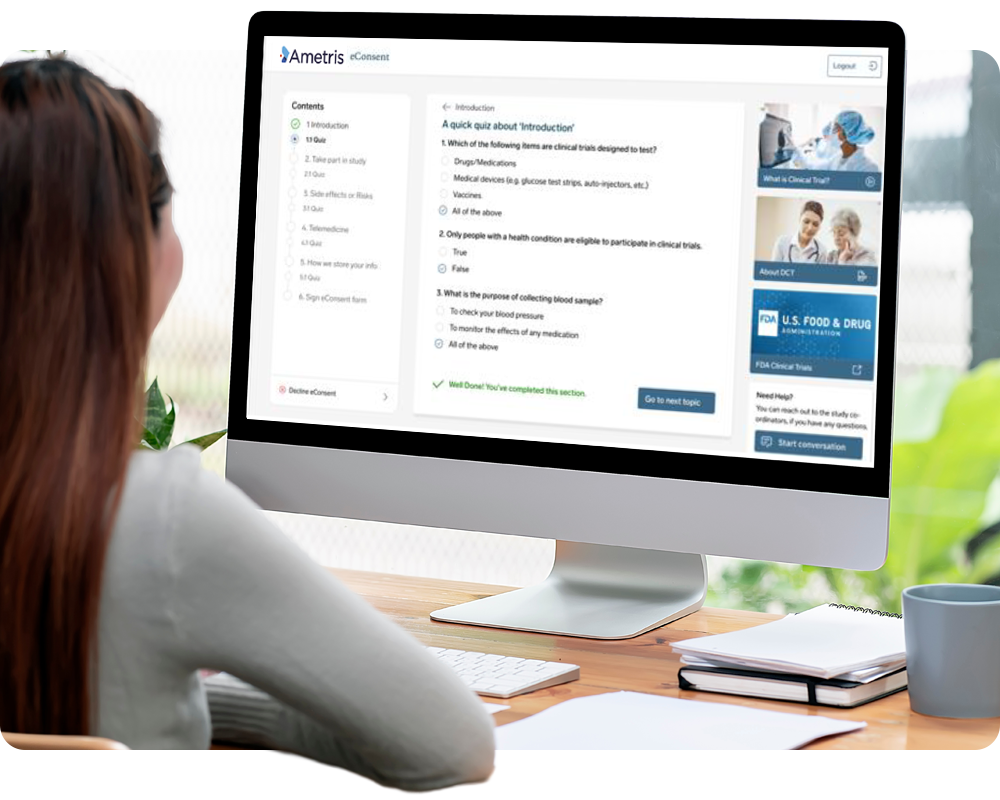
Expedite study initiation, streamline participant workflows, and improve protocol compliance with electronic informed consent. Customized for each study, the Connect platform’s integrated eConsent tool incorporates participant-friendly features like:
The Ametris digital health technology ecosystem meets rigorous global regulatory and data protection requirements, ensuring that study teams can confidently generate compliant, trustworthy, and audit-ready evidence across every stage of their research.
Visit the Ametris Compliance Center for more information.
Ecosystem of validated clinical sensors and wearables to support fit-for-purpose continuous and episodic physiologic monitoring and data capture.
Raw sensor data is transformed into validated digital measures with a comprehensive library of algorithms and expert data science support.
Centralized study oversight with tools for enrollment, onboarding tasks, and remote participant monitoring for sites and sponsors.
End-to-end DHT operations support includes project management, site training, logistics management, and clinical data operations and management.
Remote data capture and study management solutions, including eConsent, ePRO, and virtual visits, for decentralized and hybrid trials.
Patient-facing tools for onboarding, adherence monitoring, task reminders, and technical support keep participants informed and engaged.
To help study teams maximize the benefit of DHTs, Ametris has built a world-class team of data scientists, biostatisticians, clinical scientists, and engineers. Our multidisciplinary team provides end-to-end services tailoring DHT integration to your clinical hypotheses.







Have questions or need assistance? Our team is here to help. Learn more about how Ametris’s innovative solutions can support your research and patient care goals.