THERAPEUTIC EXPERTISE
Solutions for Rheumatology and Immunology Research
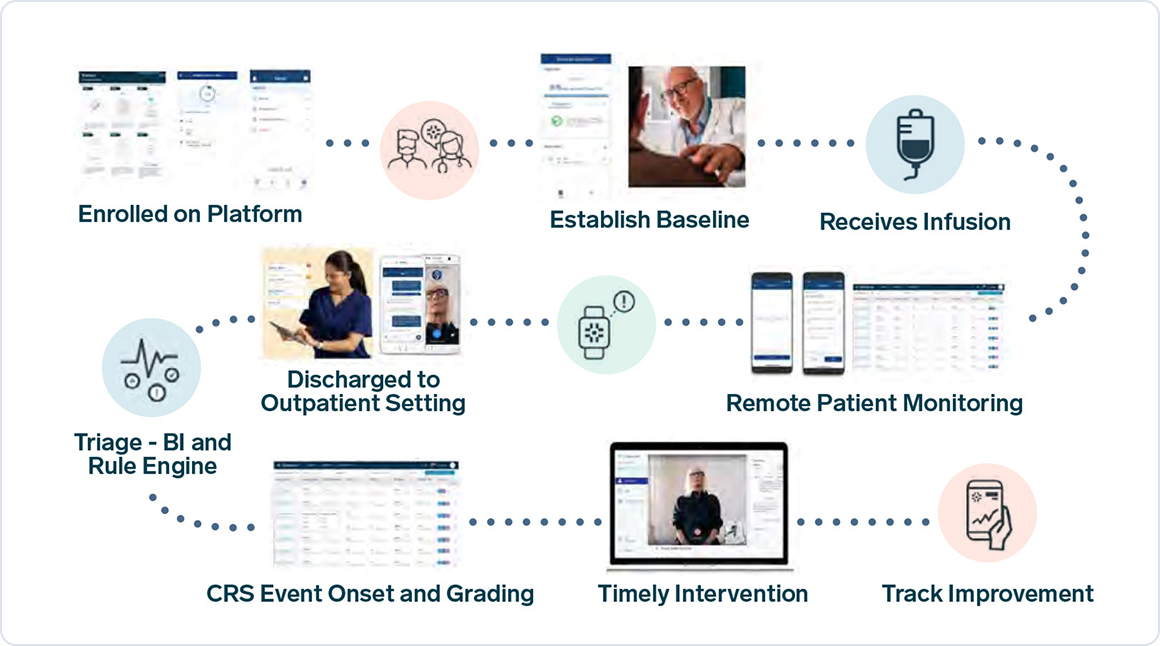
Regulatory agencies have highlighted the need to assess physical function as key efficacy endpoints in clinical research involving rheumatic and immunological conditions. Wearable digital health technologies (DHTs) capture these real-world patient-centered outcomes continuously and remotely, increasing the probability of trial success and improving management of these conditions.
Schedule a Meeting Download the Guide